Abstract
Background: Relapsed or refractory (R/R) non-Hodgkin lymphoma (NHL), remains a clinical challenge with limited second- and third-line treatment options. Patients (pts) with follicular lymphoma (FL) experiencing early relapse (ER) within 2 years of initial diagnosis and those double refractory (DR) to both rituximab and chemotherapy have particularly poor outcomes (Casulo et al. J Clin Oncol 2015; Gopal et al. N Engl J Med 2014).Avadomide (CC-122) is a cereblon modulating agent that promotes degradation of transcription factors Aiolos and Ikaros, resulting in potent antilymphoma and immunomodulatory effects on T- and NK-cell function. Phase I clinical data from the CC-122-NHL-001 study (NCT02417285) revealed promising activity with avadomide plus obinutuzumab in pts with R/R B-cell NHL (Michot et al. Blood 2017). Herein, we report results from CC-122-NHL-001 in pts with R/R FL.
Methods: CC-122-NHL-001 is a phase Ib, open-label, dose escalation/expansion study of avadomide in combination with obinutuzumab. Eligible pts were aged ≥18 y with histologically or cytologically confirmed CD20+ B-cell NHL after ≥1 prior regimen for FL/marginal zone lymphoma (MZL). Upon informed consent, pts received escalating doses of avadomide for 5 out of 7 d/wk in 28-d cycles plus a fixed dose of intravenous obinutuzumab 1000 mg on d 2, 8, and 15 of cycle 1 (C1), and d 1 of C2-8. Avadomide active ingredient in capsule (AIC) formulation at doses of 1, 2, 3, and 4 mg and avadomide formulated capsules (F6) of 3 and 4 mg were evaluated in separate cohorts. Primary endpoints included safety and tolerability, non-tolerated dose, and maximum-tolerated dose. Response was assessed using the Cheson 2007 criteria every 2 cycles to C6, every 3 cycles to C12, and every 6 cycles thereafter.
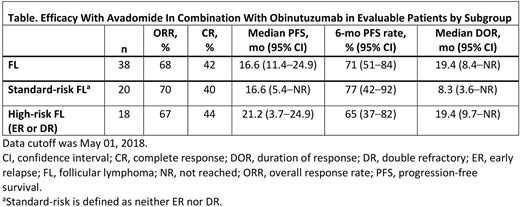
Results: As of May 1, 2018, 58 pts with R/R B-cell NHL were treated in the study, including 19 with R/R DLBCL,38 with R/R FL, and 1 with R/R MZL. Among the 38 pts with R/R FL, 18 were treated in the dose escalation phase (median of 16.5 cycles; 78% initiated ≥6 cycles) and 20 were treated in the expansion phase (median of 4 cycles; 40% initiated ≥6 cycles). Of the 38 pts, 36 pts received 3 mg of 4 mg of avadomide (F6 or AIC); 2 pts received 2 mg of avadomide (AIC). The median age among R/R FL pts was 60 y (range, 41-83), 22 pts (58%) were male, and 32 (84%) had stage III/IV disease. The median number of prior antilymphoma therapies was 3 (range, 1-8), and 12 (32%) pts had 1 prior autologous stem cell transplant. As of data cutoff, 19 pts (50%) were ongoing treatment. One pt experienced a dose-limiting toxicity, consisting of grade 4 neutropenia (avadomide 3 mg AIC). Among the 38 pts in the dose escalation/dose expansion phases, the most common (≥25%) any-grade treatment-emergent adverse events (TEAEs) were neutropenia (66%), thrombocytopenia (29%), and pyrexia (29%). The most common (≥10%) grade 3/4 TEAEs were neutropenia (58%) and thrombocytopenia (11%). Nine pts (24%) had ≥1 serious TEAE related to avadomide; only cytokine release syndrome (11%) and infusion related reactions (8%) occurred in >1 pt. Avadomide dose reduction and temporary interruption occurred in 10 pts (26%; all due to AEs), and 27 pts (71%; 25 pts due to AEs), respectively. Median duration of interruption due to AEs was 15 d (range, 2-48). The overall response rate (ORR) among all R/R FL pts (n=38) was 68%, with 16 pts (42%) achieving complete response (CR). Median duration of response was 19.4 mo (95% CI, 8.4-not reached). Median progression-free survival (mPFS) was 16.6 mo (95% CI, 11.4-24.9) with a median follow up time for PFS of 5.4 mo. Subgroup analysis examined activity of the combination in standard-risk and high-risk (ER and/or DR) FL pts (Table). Both response rates and mPFS were similar in standard-risk and high-risk FL pts (ORR: 70% vs 67%, P=0.83; CR: 40% vs 44%, P=0.78; mPFS: 16.6 mo [95% CI, 5.4-not reached] vs 21.2 mo [3.7-24.9], P=0.60). The median duration of PFS follow up in the expansion phase (n=20/38) is 3.5 mo. Updated data will be presented at ASH.
Conclusions: Avadomide given in combination with obinutuzumab was well-tolerated and demonstrated promising clinical activity, with encouraging response rates and mPFS observed in pts with R/R FL, irrespective of their disease risk status. Avadomide plus obinutuzumab may provide a new chemotherapy-free treatment option for pts with R/R FL failing standard therapies.
Vitolo:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Speakers Bureau; Sandoz: Speakers Bureau; Gilead: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kersten:Millennium/Takeda: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Kite/Gilead: Honoraria; Novartis Pharmaceuticals Corporation: Honoraria. Chiappella:Roche: Other: lecture fees; Amgen: Other: lecture fees; Nanostring: Other: lecture fees; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: lecture fees; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: lecture fees; Teva: Other: lecture fees. Zinzani:Astra Zeneca: Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Honoraria, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Speakers Bureau; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; SERVIER: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees. Salles:ACERTA: Honoraria; SERVIER: Honoraria; TAKEDA: Honoraria; GILEAD: Honoraria; CELGENE: Honoraria, Research Funding; AMGEN: Honoraria; JANSSEN: Honoraria; MERCK: Honoraria; MORPHOSYS: Honoraria; PFIZER: Honoraria; ABBVIE: Honoraria; EPIZYME: Honoraria; NOVARTIS: Consultancy, Honoraria; ROCHE: Honoraria, Research Funding. Sarmiento:Celgene Institute for Translational Research Europe: Employment, Equity Ownership. Mosulen:Celgene Institute for Translational Research Europe: Employment, Equity Ownership. Mendez:Celgene Institute for Translational Research Europe: Employment, Equity Ownership. Uttamsingh:Celgene Corporation: Employment, Equity Ownership. Pourdehnad:Celgene Corporation: Employment, Equity Ownership. Hege:Arcus Biosicences: Membership on an entity's Board of Directors or advisory committees; SITC: Membership on an entity's Board of Directors or advisory committees; Mersana: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Employment, Equity Ownership, Patents & Royalties: multiple. Li:Celgene Corporation: Employment. Nikolova:Celgene International Sarl: Employment, Equity Ownership. Ribrag:argenX: Research Funding; Infinity: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Other: travel; epizyme: Consultancy, Honoraria; NanoString Technologies: Consultancy, Honoraria; pharmamar: Other: travel; MSD: Honoraria; Gilead: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Roche: Honoraria, Other: travel; Amgen: Research Funding; Incyte Corporation: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal